Unlike traditional polymers, this structure allows MOFs to have open internal spaces with a well-defined size, which can allow some molecules to pass through while filtering out others. In addition, the presence of metals provides for interesting chemistry. The metals can serve as catalysts or preferentially bind to one molecule within a mixture.
Knowing what we know now, it all seems kind of obvious that this would work. But when Robson started his work at the University of Melbourne, the few people who thought about the issue at all expected that the molecules he was building would be unstable and collapse.
The first MOF Robson built used copper as its metal of choice. It was linked to an organic molecule that retained its rigid structure through the presence of a benzene ring, which doesn’t bend. Both the organic molecule and the copper could form four different bonds, allowing the structure to grow by doing the rough equivalent of stacking a bunch of three-sided pyramids—a conscious choice by Robson.
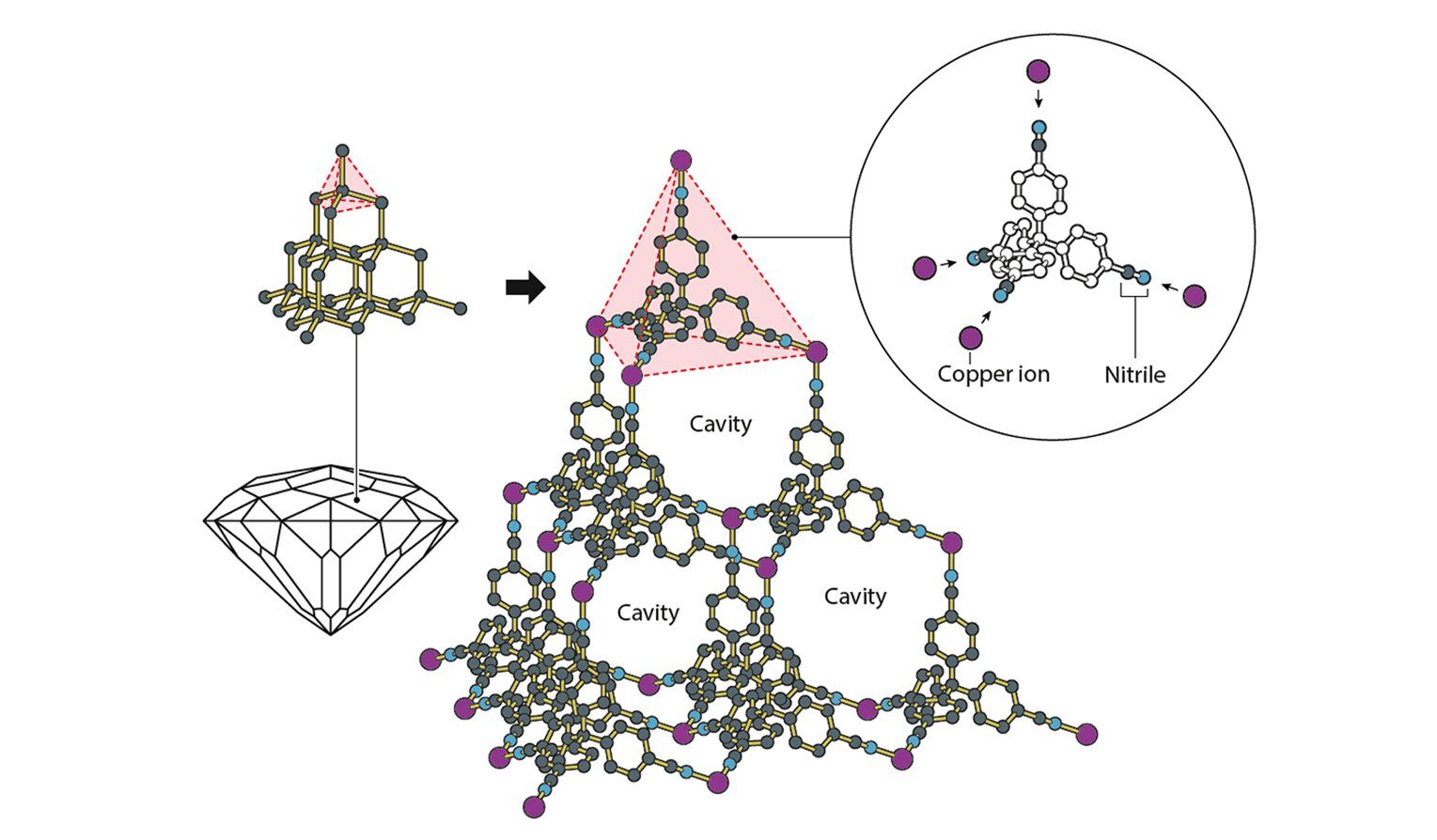
The world’s first MOF, synthesized by Robson and his colleagues. Credit: Johan Jarnestad/The Royal Swedish Academy of Sciences
In this case, however, the internal cavities remained filled by the solvent in which the MOF was formed. But the solvent could move freely through the material. Still, based on this example, Robson predicted many of the properties that have since been engineered into different MOFs: the ability to retain their structure even after solvents are removed, the presence of catalytic sites, and the ability of MOFs to act as filters.
Expanding the concept
All of that might seem a very optimistic take for someone’s first effort. But the measure of Robson’s success is that he convinced other chemists of the potential. One was Susumu Kitagawa of Kyoto University. Kitagawa and his collaborators built a MOF that had large internal channels that extended the entire length of the material. Made in a watery solution, the MOF could be dried out and have gas flow through it, with the structure retaining molecules like oxygen, nitrogen, and methane.