Fragments of bird flu virus genome found in pasteurized milk, FDA says
Milk testing —
The test cannot tell if the virus is live. The FDA still assess milk supply as safe.
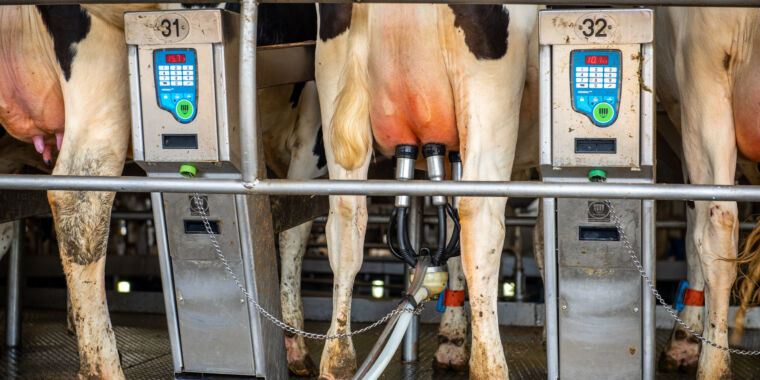
Enlarge / Cows being milked
The Food and Drug Administration on Tuesday announced that genetic fragments from the highly-pathogenic avian influenza virus H5N1 have been detected in the pasteurized, commercial milk supply. However, the testing completed so far—using quantitative polymerase chain reaction (qPCR)—only detects the presence of viral genetic material and cannot tell whether the genetic material is from live and infectious viral particles or merely remnants of dead ones killed by the pasteurization process.
Testing is now ongoing to see if viable, infectious H5N1 can be identified in milk samples.
So far, the FDA still believes that the milk supply is safe. “To date, we have seen nothing that would change our assessment that the commercial milk supply is safe,” the agency said in a lengthy explanation of the finding and ongoing testing.
H5N1 made its startling jump to US dairy cows recently, with the first ever documented cases in a Texas herd confirmed on March 25. It has spread widely since then with at least 32 herds in eight states now known to be infected. The unexpected spread to bovines has raised fears that the virus is evolving to infect mammals more efficiently, and so poses a heightened risk of spread to and among humans.
But amid the alarming outbreak among the country’s dairy herds, federal agencies have appeared confident that the virus poses little risk to no risk to the safety of the milk supply.
“At this time, there continues to be no concern that this circumstance poses a risk to consumer health, or that it affects the safety of the interstate commercial milk supply because products are pasteurized before entering the market” the FDA wrote in an FAQ published Friday. “Pasteurization has continually proven to inactivate bacteria and viruses, like influenza, in milk.”
In the announcement Tuesday, the FDA also highlighted that multiple studies have shown that the pasteurization process for eggs, which uses lower temperatures than what is used for milk, is effective at inactivating H5N1.
Nevertheless, the FDA, along with the Centers for Disease Control and Prevention and the US Department of Agriculture, have continued to investigate potential risks, including establishing whether pasteurization can inactivate this specific virus. The FDA noted in its announcement Tuesday that, while pasteurization is expected to kill the virus, pasteurization is “different than complete sterilization.”
As such, it carried out the qPCR tests, expecting it might find some genetic fragments in the pasteurized milk because virus has been detected in raw milk. “Based on available information, pasteurization is likely to inactivate the virus, however the process is not expected to remove the presence of viral particles,” the FDA explained. “Therefore, some of the samples collected have indicated the presence of HPAI [Highly Pathogenic Avian Influenza] using quantitative polymerase chain reaction (qPCR) testing.”
The FDA did not indicate how many samples it has tested, where the samples were collected from, or the level of viral genetic material the samples contained.
The agency is now working on assessing whether it can identify if any virus particles are infectious using egg inoculation tests, which are considered a gold-standard for determining viral viability. It added that it will release results from those tests and others in “the next few days to weeks.”
“[W]e take this current situation and the safety of the milk supply very seriously. We recognize the importance of releasing further, actionable information,” the FDA said.
Meanwhile, the agency reported that the CDC’s food safety group has been closely monitoring emergency department data and flu testing data for any unusual trends in flu-like illness, flu, or conjunctivitis, which could indicate spread of H5N1 to people. “To date, surveillance systems do not show any unusual trends or activity,” the FDA said.
Fragments of bird flu virus genome found in pasteurized milk, FDA says Read More »