Physics of the perfect cacio e pepe sauce
The trick: Add corn starch separately to make the sauce rather than using pasta water.
Cacio e pepe is an iconic pasta dish that can be frustratingly difficult to make Credit: Simone Frau
Nobody does pasta quite like the Italians, as anyone who has tasted an authentic “pasta alla cacio e pepe” can attest. It’s a simple dish: just tonnarelli pasta, pecorino cheese, and pepper. But its simplicity is deceptive. Cacio e pepe (“cheese and pepper”) is notoriously challenging to make because it’s so easy for the sauce to form unappetizing clumps with a texture more akin to stringy mozzarella rather than being smooth and creamy.
A team of Italian physicists has come to the rescue with a foolproof recipe based on their many scientific experiments, according to a new paper published in the journal Physics of Fluids. The trick: using corn starch for the cheese and pepper sauce instead of relying on however much starch leaches into the boiling water as the pasta is cooked.
“A true Italian grandmother or a skilled home chef from Rome would never need a scientific recipe for cacio e pepe, relying instead on instinct and years of experience,” the authors wrote. “For everyone else, this guide offers a practical way to master the dish. Preparing cacio e pepe successfully depends on getting the balance just right, particularly the ratio of starch to cheese. The concentration of starch plays a crucial role in keeping the sauce creamy and smooth, without clumps or separation.”
There has been a surprising amount of pasta-related physics research in recent years, particularly around spaghetti—the mechanics of slurping the pasta into one’s mouth, for instance, or spitting it out (aka, the “reverse spaghetti problem”). The most well-known is the question of how to get dry spaghetti strands to break neatly in two rather than three or more scattered pieces. French physicists successfully explained the dynamics in an Ig Nobel Prize-winning 2006 paper. They found that, counterintuitively, a dry spaghetti strand produces a “kick back” traveling wave as it breaks. This wave temporarily increases the curvature in other sections, leading to many more breaks.
In 2020, physicists provided an explanation for why a strand of spaghetti in a pot of boiling water will start to sag as it softens before sinking to the bottom of the pot and curling back on itself in a U shape. Physicists have also discovered a way to determine if one’s spaghetti is perfectly done by using a simple ruler (although one can always use the tried-and-true method of flinging a test strand against the wall). In 2021, inspired by flat-packed furniture, scientists came up with an ingenious solution to packaging differently shaped pastas: ship them in a flat 2D form that takes on the final 3D shape when cooked, thanks to carefully etched patterns in the pasta.
And earlier this year, physicists investigated how adding salt to a pasta pot to make it boil faster can leave a white ring on the bottom of the pot to identify factors leading to the perfect salt ring. They found that particles released from a smaller height fall faster and form a pattern with a clean central region. Those released from a greater height take longer to fall to the bottom, and the cloud of particles expands radially until the particles are far enough apart not to be influenced by the wakes of neighboring particles, such that they no longer form a cloud. In the latter case, you end up with a homogeneous salt ring deposit.
Going through a phase (separation)
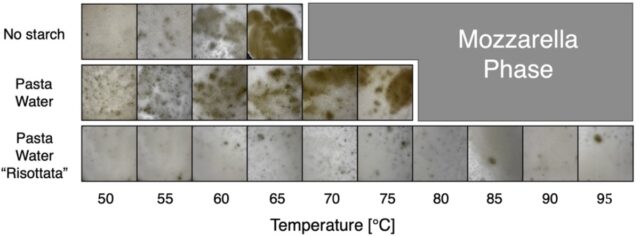
Comparing the effect of water alone, pasta water that retains some starch, and pasta water “risottata.” Credit: G. Bartolucci et al., 2025
So it shouldn’t be the least bit surprising that physicists have now turned their attention to the problem of the perfect cacio e pepe sauce. The authors are well aware that they are treading on sacred ground for Italian traditionalists. “I hope that eight Italian authors is enough [to quell skepticism],” co-author Ivan Di Terlizzi of the Max Planck Institute for the Physics of Complex Systems told The New York Times back in January. (An earlier version of the paper was posted to the physics preprint arXiv in January, prompting that earlier coverage.)
Terlizzi and his fellow author are all living abroad and frequently meet for dinner. Cacio e pepe is among their favorite traditional dishes to make, and as physicists, they couldn’t help but want to learn more about the unique physics of the process, not to mention “the more practical aim to avoid wasting good pecorino,” said Terlizzi. They focused on the separation that often occurs when cheese and water are mixed, building on earlier culinary experiments.
As the pasta cooks in boiling water, the noodles release starch. Traditionally, the chef will extract part of the water and starch solution—which is cooled to a suitable temperature to avoid clumping as the cheese proteins “denaturate”—and mix it with the cheese to make the sauce, adding the pepper last, right before serving. But the authors note that temperature is not the only factor that can lead to this dreaded “mozzarella phase.”
According to the authors, if one tries to mix cheese and water without any starch, the clumping is more pronounced. There is less clumping with water containing a little starch, like water in which pasta has been cooked. And when one mixes the cheese with pasta water “risottata”—i.e., collected and heated in a pan so enough water evaporates that there is a higher concentration of starch—there is almost no clumping.
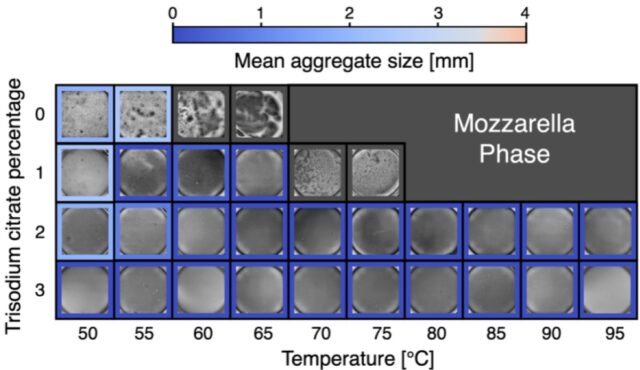
Effect of trisodium citrate on the stability of cacio e pepe sauce. Credit: G. Bartolucci et al., 2025
So starch plays a crucial role in the process of making cacio e pepe. The authors devised a set of experiments to scientifically investigate the phase behavior of water, starch, and cheese mixed together in various concentrations and at different temperatures. They primarily used standard kitchen tools to make sure home cooks could recreate their results (although not every kitchen has a sous vide machine). This enabled them to devise a phase diagram of what happens to the sauce as the conditions change.
The authors found that the correct starch ratio is between 2 to 3 percent of the cheese weight. Below that, you get the clumping phase separation; above that, and the sauce “becomes stiff and unappetizing as it cools,” they wrote. Pasta water alone contains too little starch. Using pasta water “risottata” may concentrate the starch, but the chef has less control over the precise amount of starch. So the authors recommend simply dissolving 4 grams of powdered potato or corn starch in 40 grams of water, heating it gently until it thickens—a transition known as starch gelatinization—and combining that gel with the cheese. They also recommend toasting the black pepper briefly before adding it to the mixture to enhance its flavors and aromas.
They ran the same set of experiments using trisodium citrate as an alternative stabilizer, which is widely used in the food industry as an emulsifier—including in the production of processed cheese, since it enhances smoothness and prevents unwanted clumping, exactly the properties one desires for a perfect cacio e pepe sauce. The trisodium citrate at concentrations above 2 percent worked just as well at avoiding the mozzarella phase, “though at a cost of deviating from strict culinary tradition,” the authors concluded. “However, while the sauce stabilization is more efficient, we found the taste of the cheese to be slightly blunted, likely due to the basic properties of the salt.”
The team’s next research goal is to conduct similar experiments with making pasta alla gricia—basically the same as cacio e pepe, with the addition of guanciale (cured pork cheek). “This recipe seems to be easier to perform, and we don’t know exactly why,” said co-author Daniel Maria Busiello, Terlizzi’s colleague at the Dresden Max Planck Institute. “This is one idea we might explore in the future.”
DOI: Physics of Fluids, 2025. 10.1063/5.0255841 (About DOIs).
Jennifer is a senior writer at Ars Technica with a particular focus on where science meets culture, covering everything from physics and related interdisciplinary topics to her favorite films and TV series. Jennifer lives in Baltimore with her spouse, physicist Sean M. Carroll, and their two cats, Ariel and Caliban.
Physics of the perfect cacio e pepe sauce Read More »