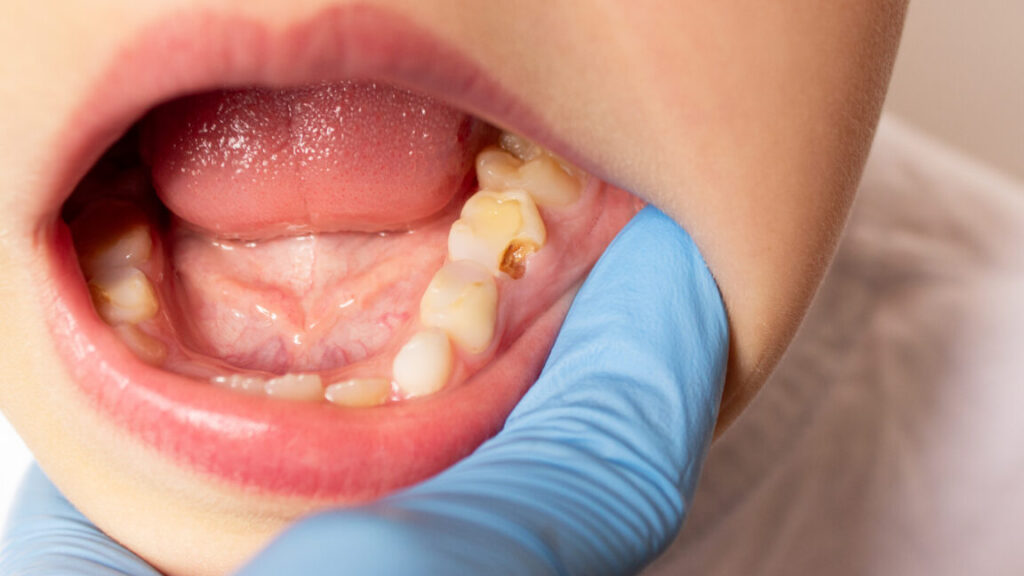
RFK Jr.’s fluoride ban would ruin 25 million kids’ teeth, cost $9.8 billion
In all, the increased decay and boosted dental costs would disproportionately affect children who are in low-income families, in rural areas, and/or on public health insurance.
The study’s findings are likely unsurprising to those in the public health community, who have consistently supported fluoridation. The practice, however beneficial, has a long history of being under attack. After its introduction in the US in 1945, conspiracy theorists claimed fluoridation was a communist plot and a form of government mind control. More recently, critics have claimed that fluoridation lowers IQ.
The data linking water fluoridation to low IQ is controversial. Many of the studies on the topic are of poor quality and have numerous confounding factors and flawed methods. Many compare IQ levels in communities in China and other countries, where there are areas with water that is naturally high in fluoride—much, much higher than what is intentionally added to US water. Further, a federal meta-analysis—a type of study that aggregates and reanalyzes data from independent studies—has been plagued by criticism for bias, poor statistical methods, and a lack of data transparency.
But despite the controversy, one thing is clear in all the data and debate: Any possible association with low IQ and fluoridation only occurs at excessive levels—levels more than twice the amount used in the US and recommended by the US Centers for Disease Control and Prevention. The CDC recommendation for water fluoridation levels is 0.7 mg/L, while potential harms are not observed until water levels exceed 1.5 mg/L. Some areas in China have natural levels as high as 11.8 mg/L.
The authors of the new study conclude that, at current US levels, the benefits are clear.
“These findings suggest that, despite the potential harms of excessive fluoride exposure, fluoridation at safe levels offers both individual and societal benefits that would be at risk.”
RFK Jr.’s fluoride ban would ruin 25 million kids’ teeth, cost $9.8 billion Read More »