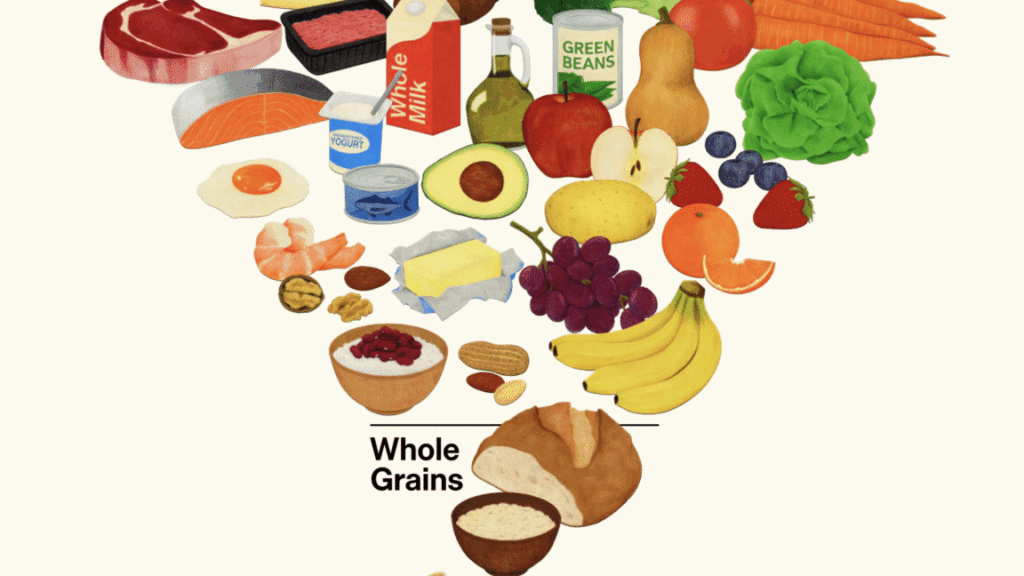RFK Jr.’s dietary guidance: Food funnel features slab of red meat, butter
Earning some praise from outside experts, including the American Medical Association, the new guidelines are the first iteration to directly address highly processed foods. While emphasizing “whole, nutrient-dense foods,” it aims for a “dramatic reduction in highly processed foods laden with refined carbohydrates, added sugars, excess sodium, unhealthy fats, and chemical additives.”
While the guidelines don’t provide a clear definition of what constitutes highly processed foods or how consumers can identify them, they do offer some broad examples at various points, including store-bought “chips, cookies, and candy,” and “white bread, ready-to-eat or packaged breakfast options, flour tortillas, and crackers.”
New triangle
In an effort to steer Americans to healthy choices, the new guidance unveils a new(ish) visual aid—a food pyramid that is upside-down, thus resembling a funnel.
The move at least explains a puzzling trend: Over the past year, Kennedy and other Trump administration officials have repeatedly made reference to the food pyramid—though only to mock and scorn it, often with inaccuracies.
“The dietary guidelines that we inherited from the Biden administration were 453 pages long,” Kennedy said in August, referring to the 2020–2025 guidelines, which are 164 pages long. “They were driven by the same commercial impulses that put Froot Loops at the top of the food pyramid.”
On Wednesday’s unveiling of the new guidelines, Food and Drug Administration Commissioner Marty Makary lamented that, “for decades, we’ve been fed a corrupt food pyramid.”
Not only were Froot Loops never listed on a food pyramid, no food pyramid has been included in federal dietary guidelines for over a decade, raising the question of why the administration was repeatedly attacking a defunct polyhedron. The original food pyramid was introduced in 1992, significantly revised in 2005, and ditched entirely in 2011. Since then, the guidelines have used MyPlate as a visual aid, intended to provide a simplistic depiction of the foods people should eat, in their recommended proportions, on a plate.
RFK Jr.’s dietary guidance: Food funnel features slab of red meat, butter Read More »